In late 2023, Mirus Bio added RevIT™ AAV Enhancer to its established portfolio of reagents and kits for viral vector production to meet calls for more virus per transfection for any AAV construct.
![]()
Across the industry, we were hearing about processes that had worked efficiently for producing one drug candidate but were falling short during scaleup or when applied to a different serotype or GOI. To address this prevalent problem, we designed RevIT™ with the idea that it could be plugged into any production platform to amplify yields of any AAV construct.
In this report, we describe the RevIT™ AAV Enhancer discovery process and how RevIT™ AAV Enhancer significantly increases the number of AAV genomes produced across multiple production platforms and serotypes.
![]()
Since such high AAV titers could be achieved by simply incorporating RevIT™ AAV Enhancer during transfection, we wondered if we could still attain high titers while transfecting less plasmid DNA. We titrated the total amount of plasmid DNA used in transfection, in the absence or presence of RevIT™ AAV Enhancer, and titered the AAV5 that was produced (Figure 1). (For additional data and for other serotypes, please see the white paper.)
Remarkably, addition of RevIT™ AAV Enhancer approximately doubled AAV titers at all amounts of DNA used for transfection! In other words, the same or even greater titer could be achieved with a fraction of the DNA in the presence of RevIT™ AAV Enhancer. Interestingly, we also observed that the percentage of full AAV particles produced was improved when less DNA was used in transfection. Thus, by using RevIT™ AAV Enhancer, we were able to produce high-titer and high-quality AAV using less plasmid DNA.
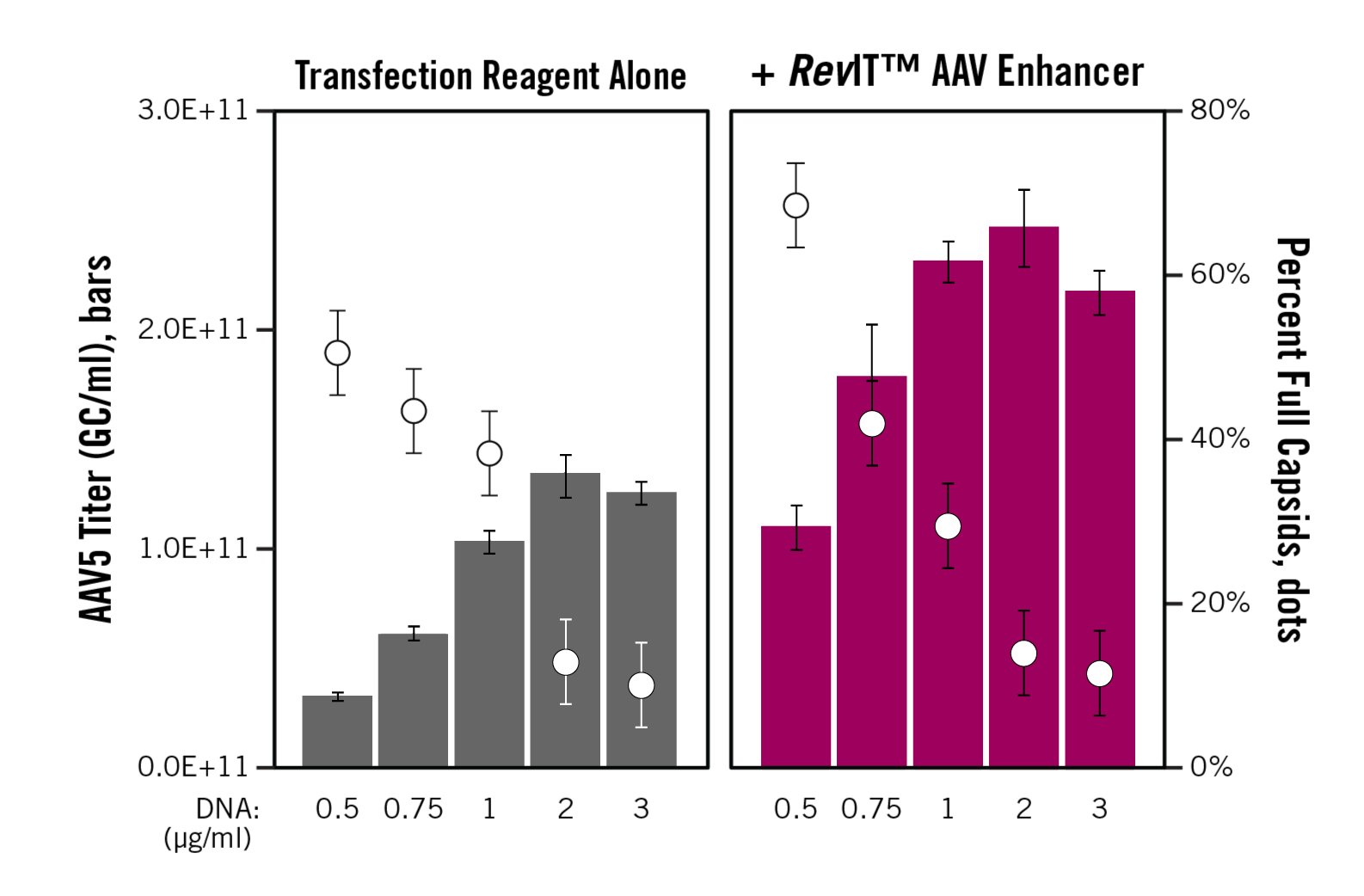
Figure 1. RevIT™ AAV Enhancer enables using less plasmid DNA to achieve high titers and percent full capsids. Viral Production Cells 2.0 (3E6 cells/ml of culture, Gibco) cultured in Viral Production Medium (Gibco) in 6-well plates were transfected with TransIT-VirusGEN® Reagent (3 µl/ml of culture) and the indicated concentration of total plasmid DNA (pAAV-hrGFP and pHelper (Agilent), pALD-AAV5 (Aldevron)). If used, RevIT™ AAV Enhancer was added to the transfection mixture at 0.5 µl/ml of culture. AAV was harvested at 72 hours post-transfection. Genome copies were determined by dPCR using primers and a probe targeting the CMV promoter. Capsids were determined using the AAV5 Titration ELISA (Progen). The percent of full capsids is calculated by taking the percentage of genome copies to capsids. The error bars represent the range of duplicate wells.
We also examined how RevIT™ AAV Enhancer performed in scaled-up AAV production processes (Figure 2). In this experiment, we produced AAV8 in cells cultured in shake flasks and bioreactors using the same, but volume-scaled, transfection complexes between formats. Again, by using RevIT™ AAV Enhancer we were able to produce more AAV with a higher percentage of full capsids, using half the amount of plasmid DNA! Furthermore, the results were consistent between transfection of 30-ml cultures in shake flasks and 2-L cultures in bioreactors, suggesting RevIT™ AAV Enhancer can seamlessly transition from R&D to commercial manufacturing scales.
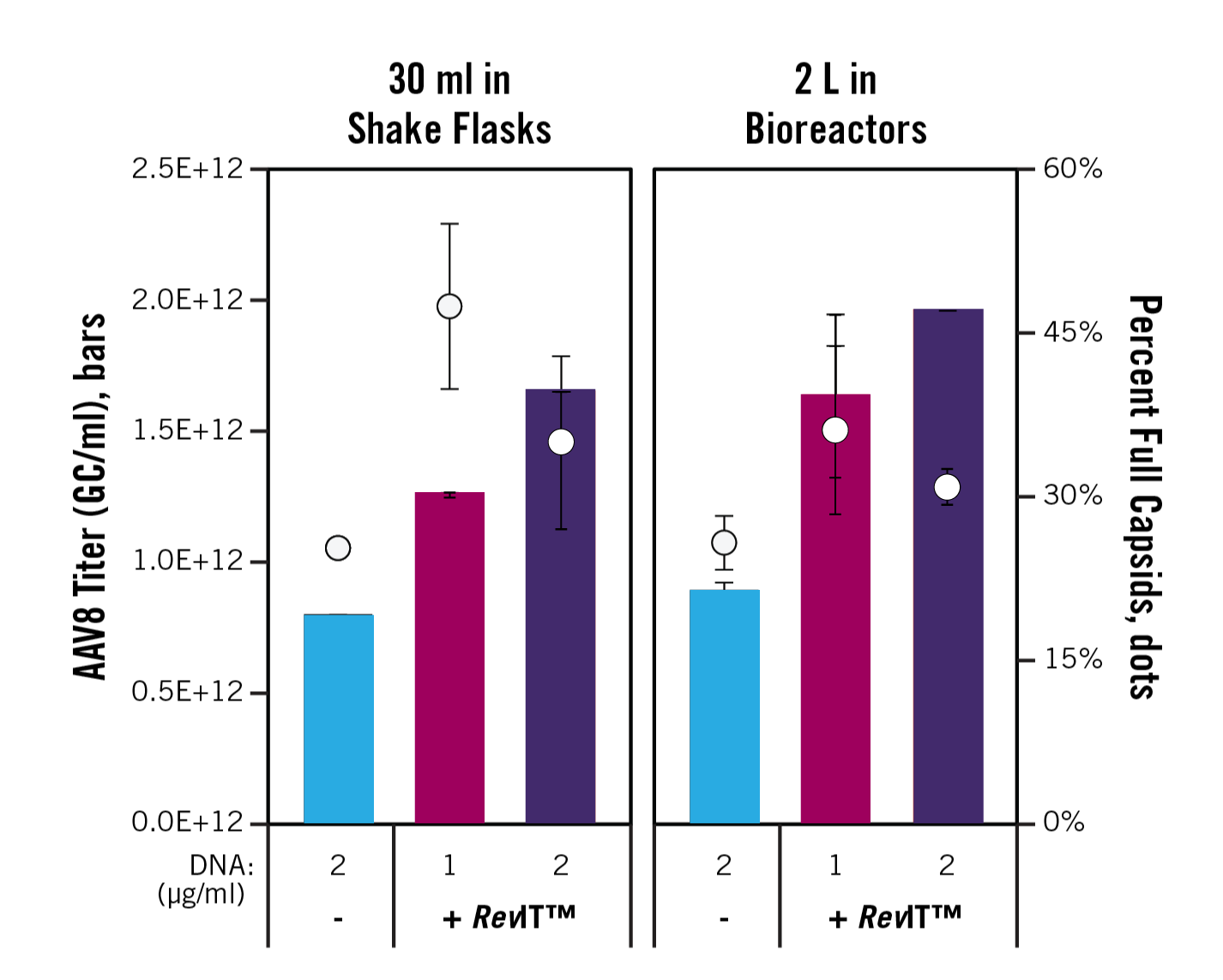
Figure 2. Consistent, high productivity is observed when using RevIT™ AAV Enhancer in both shake flasks and bioreactors. Viral Production Cells 2.0 (3E6 cells/ml of culture, Gibco) cultured in BalanCD HEK293 Medium (Irvine Scientific) supplemented with GlutaMAX (Gibco) were transfected with TransIT-VirusGEN® Reagent (3 µl/ml of culture) and the indicated concentration of total plasmid DNA (pALD-ITR-WPRE-GFP and pALD-HELP (Aldevron), AAV8 Rep-Cap plasmid (GeneMedi)). If used, RevIT™ AAV Enhancer was added to the transfection mixture at 1 µl/ml of culture. AAV was harvested at 72 hours post-transfection. Genome copies were determined by dPCR using primers and a probe targeting the CMV promoter. Capsids were determined using the AAV8 Titration ELISA (Progen). The percent of full capsids is calculated by taking the percentage of genome copies to capsids. The error bars represent the range of duplicate flasks for the 30-ml cultures; the error bars represent the range of duplicate samples pulled from the same bioreactor approximately 20 minutes apart for the 2-L cultures.
In conclusion, with little to no additional modification to existing AAV production workflows, AAV manufacturers can expect BIG process improvements with RevIT™ AAV Enhancer. RevIT™ AAV Enhancer enables using less DNA to achieve higher AAV titers and percentage of full capsids per production run. Hopefully, these process improvements and cost savings translate to more affordable and accessible gene therapies.
Ready to rev-up your AAV titers? Email revit@mirusbio.com to get in touch with Mirus!
Explore Related Info & Links
- Download this white paper for more data on RevIT™ AAV Enhancer
- Check out RevIT™ AAV Enhancer
- Check out TransIT-VirusGEN® Transfection Reagent
- Learn more about transfection reagents for producing gene and cell therapies here
The TransMission
Feedback or questions? We’d love to hear from you. Email techsupport@mirusbio.com or call us at 888.530.0801.