Mirus Bio receives ISO 13485:2016 certification, underscoring the quality of processes used to support GMP product portfolio. Read more
Mirus Bio receives ISO 13485:2016 certification, underscoring the quality of processes used to support GMP product portfolio. Read more
Co-transfection or co-delivery of multiple nucleic acids – either similar or different in nature is a technique routinely employed by cell biologists to address increasingly complex experimental design and applications. Common examples of nucleic acid co-transfection are as follows:
Most transfection reagents are designed for efficient delivery of a single nucleic acid type such as plasmid DNA, siRNA, mRNA, etc. Therefore, co-transfecting multiple nucleic acids of the same kind such as multiple plasmid DNAs or siRNAs is relatively straightforward as long as the ratio of the amount of transfection reagent to total nucleic acid is maintained. Co-delivery of completely different nucleic acid types can be quite tricky depending on the combination.
Co-transfection of different nucleic acid types such as large plasmid DNA and smaller siRNA/miRNAs can be challenging primarily due to the size and charge difference between these nucleic acid molecules. The charge of the nucleic acid molecule impacts the charge ratio of the transfection complexes and their formation and uptake by the mammalian cell membrane. Due to this reason, transfection reagents that can deliver smaller nucleic acids such as siRNA/miRNA to the cytoplasm efficiently are not always successful in plasmid DNA delivery to the nucleus. Unique polymeric formulations such as the TransIT-X2® Dynamic Delivery System are very effective nucleic acid condensing agents. These polymers provide positive charged side groups that can interact efficiently with the negative backbone of DNA and siRNA to form tight complexes that have a net positive surface charge. Once formed and condensed the transfection complexes are taken up very efficiently via endocytosis and nucleic acid delivery to their respective functional locations: the nucleus (DNA) and cytoplasm (siRNA/miRNA). Learn more about how the TransIT-X2® Dynamic Delivery System enables co-transfection of plasmid DNA and siRNA here.
For transfection reagent recommendations to deliver various different combinations of nucleic acids, please refer to the table here. If a single transfection reagent is not suitable for a specific co-delivery application, then separate transfection complexes can be prepared using the optimal transfection reagent for each nucleic acid and added to the cells at the same time. As long as the individual transfection protocols are compatible, this method ensures uptake of all nucleic acids by the cell simultaneously.
Co-transfection of multiple nucleic acids is a technique increasingly employed by researchers. Applications that popularly utilize multiple plasmid DNA co-transfection are virus production, protein-protein interaction studies, stable cell line generation, or simple addition of reporter DNA constructs to normalize experimental output. Multiple siRNA molecules can be transfected for pooled siRNA transfection. Recently, co-delivery of multiple mRNA transcripts encoding differentiation factors has been employed for non-integrative stem cell reprogramming applications. One example utilizing co-transfection of multiple plasmid DNA molecules for virus production is illustrated below.
For a detailed protocol for co-transfecting multiple plasmid DNAs, please click on the following link: Transient DNA Co-Transfection Protocol in a 6-Well Plate using TransIT-X2® System
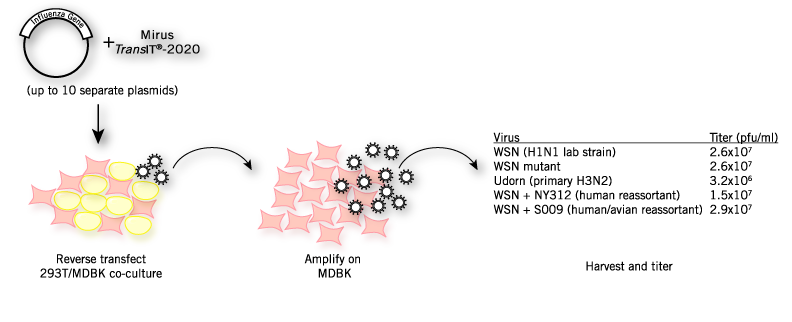
Rescue of Influenza Virus by Reverse Transfection With TransIT®-2020 Transfection Reagent. 293T and MDBK cells are reverse transfected with up to 10 separate influenza virus rescue plasmids per experiment. Reagent:total DNA complexes are prepared with TransIT®-2020 Transfection Reagent at a 3:1 ratio of reagent to total DNA. Virus-containing supernatants are harvested 48-96 hr post-transfection. Titers from transfected cells can be as high as 107 pfu/ml. Rescued virus is then amplified on MDBK cells to increase titer and total yield. Viral stocks are subsequently titered by plaque assay on MDCK cells. Titers from representative rescue experiments with different viral strains and reassortants are shown. This experimental strategy is adapted from Neumann, et al. 2005 PNAS.
Data courtesy of Andrew Mehle, University of Wisconsin-Madison
All of the TransIT® broad-spectrum and cell-type specific DNA transfection reagents can be used for co-transfecting multiple plasmid DNAs. For siRNA co-transfection, any of the TransIT® broad-spectrum siRNA transfection reagents can be used. Depending on the cell type being transfected, one reagent may have superior performance over others; for cell-type specific recommendations, please consult the Reagent Agent® transfection database. Multiple mRNAs can be transfected using the broad spectrum TransIT®-mRNA Transfection Kit.
| Nucleic Acid(s) to be Co-transfected | Transfection Reagent | Application(s) |
|---|---|---|
| Multiple plasmid DNAs | Option 1: TransIT-X2® Dynamic Delivery System Option 2: TransIT®-2020 Transfection Reagent Option 3: TransIT®-LT1 Transfection Reagent | Virus production Dual reporter assays Stable cell line generation |
| Multiple siRNA/miRNAs | Option 1: TransIT-X2® Dynamic Delivery System Option 2: TransIT-TKO® Transfection Reagent Option 3: TransIT-siQUEST® Transfection Reagent | Pooled siRNA transfection |
| Multiple mRNAs | TransIT®-mRNA Transfection Kit | Stem cell reprogramming |
| Multiple oligos | TransIT®-Oligo Transfection Reagent | Pooled oligo transfection |
In addition to general user protocol guidelines such as optimal cell confluence, high quality nucleic acid, etc., following guidelines should be considered for co-transfection experiments of similar nucleic acid molecules.
Outlined below is an easy-to-follow co-transfection protocol for multiple plasmid DNAs using TransIT-X2® Dynamic Delivery System in a 6-well format. TransIT-X2® Dynamic delivery system can also be used to co-deliver multiple siRNAs; additionally, it also enables efficient co-delivery of plasmid DNA and siRNA. For additional details, please refer to the user protocol (PDF) of TransIT-X2® System.
For co-delivery tips for other nucleic acid molecules such as mRNA, oligos, etc., please contact techsupport@mirusbio.com.
* The above protocol can be used when using other DNA transfection reagents such as TransIT®-2020 and TransIT®-LT1. However, depending on the transfection reagent and the cell type used for co-transfection, the ratio of the transfection reagent: total DNA may need to be re-optimized.
Co-transfection of plasmid DNA and siRNA/miRNA is a technique popularly used by cell biologists, for applications such as knockdown rescue or siRNA/miRNA mediated knockdown of gene expression from a plasmid DNA that is being delivered to the cell. Generally, transfection reagents that can deliver smaller nucleic acids such as siRNA/miRNA to the cytoplasm efficiently are not as successful in plasmid DNA delivery to the nucleus. This is due to the size/charge difference between siRNA/miRNA and plasmid DNA, and the subcellular location for those molecules to act (cytoplasm for siRNA/miRNA and nucleus for plasmid DNA). The new TransIT-X2® Dynamic Delivery System affords cutting-edge co-delivery of plasmid DNA and siRNA/miRNA due to its novel, non-liposomal, polymeric nature.
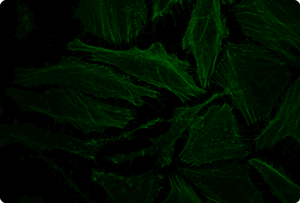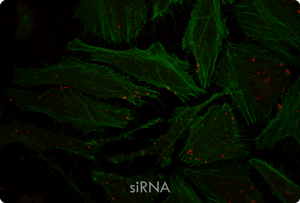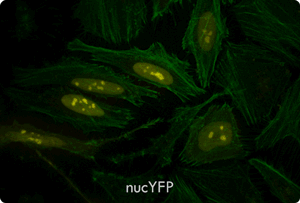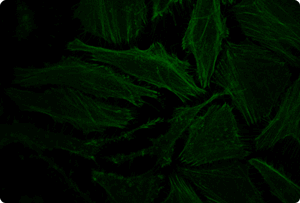
Functional Co-transfection of Plasmid DNA and siRNA Using the TransIT-X2® Dynamic Delivery System. TransIT-X2® Dynamic Delivery System was used to transfect plasmid Cy®5 labeled DNA encoding nuclear YFP and Cy®3 labeled siRNA into HeLa cells. Transfection was performed in 6-well plates with Poly-L-Lysine (PLL) coated coverslips using 4 µl of TransIT-X2® to deliver 2 µg of DNA and 25 nM siRNA (2:1 reagent:DNA ratio). Actin cytoskeleton was stained using Alexa Fluor® 350 Phalloidin. Images (63X) were captured at 24 hours post-transfection using a Nikon A1R confocal microscope. Image key: yellow (nuclear YFP), blue (Cy®5 labeled DNA), red (Cy®3 labeled siRNA), green (actin cytoskeleton).
Outlined below is an easy-to-follow co-transfection protocol for plasmid DNA and siRNA using TransIT-X2® Dynamic Delivery System in a 6-well format. For other tissue culture formats, refer to the user protocol (PDF). This protocol can also be followed when co-transfecting plasmid DNA and miRNA by simply substituting miRNA for siRNA.
Co-delivery of plasmid DNA and mRNA is a co-transfection technique employed in specific applications such as creating gene insertion using Zinc Finger Nucleases (ZFNs). Following is a straight-forward protocol using TransIT®-mRNA Transfection Kit adapted from Sigma’s user manual for CompoZr® Targeted Integration Kit-AAVS (Sigma Aldrich) (PDF). While this co-transfection protocol has been reported to work with TransIT®-mRNA Transfection Kit, other Mirus’ DNA transfection reagents deliver DNA more efficiently. For optimal co-delivery of plasmid DNA and mRNA, we recommend making separate DNA transfection complexes using one of Mirus’ broad spectrum DNA transfection reagents such as TransIT-X2® Dynamic Delivery System followed by separate mRNA transfection complexes using TransIT®-mRNA Transfection Kit. This order of addition is due to the time-sensitive nature of mRNA transfection complex formation using TransIT®-mRNA Transfection Kit. Detailed protocol links for each approach are listed below.
NOTE: Depending on the application, the optimal reagent levels to use for RNA and DNA co-transfection should be tested by including a range of TransIT®-mRNA Reagent levels (2.5,5, and 7.5 μl) with a range of mRNA Boost Reagent amounts (2.5, 5, and 7.5 μl). The amounts of RNA and DNA themselves might also need to be optimized to ensure successful co-delivery.
NOTE: While the above protocol has been reported to work with TransIT®-mRNA Transfection Kit, we recommend making separate DNA transfection complexes using one of Mirus’ broad spectrum DNA transfection reagents such as TransIT-X2® Dynamic Delivery System followed by separate mRNA transfection complexes using TransIT®-mRNA Transfection Kit. This allows optimal co-delivery of plasmid DNA and mRNA; a detailed protocol is listed below.
Different TransIT® Transfection Reagents are recommended depending on the combinations of nucleic acids being delivered. All of the following reagent recommendations are of broad spectrum formulations that are chemically distinct. Depending on the cell type being transfected, one reagent may have superior performance over others; for cell-type specific recommendations, please consult the Reagent Agent® transfection database or contact techsupport@mirusbio.com
| Nucleic Acid(s) to be Co-transfected | Transfection Reagent |
|---|---|
| Multiple plasmid DNAs | Option 1: TransIT-X2® Dynamic Delivery System Option 2: TransIT®-2020 Transfection Reagent Option 3: TransIT®-LT1 Transfection Reagent |
| Multiple siRNAs | Option 1: TransIT-X2® Dynamic Delivery System Option 2: TransIT-TKO® Transfection Reagent Option 3: TransIT-siQUEST® Transfection Reagent |
| Plasmid DNA and siRNA | TransIT-X2® Dynamic Delivery System |
| Multiple mRNAs | TransIT®-mRNA Transfection Kit |
| Plasmid DNA and mRNA | TransIT-X2® Dynamic Delivery System and TransIT®-mRNA Transfection Kit |
| Multiple oligonucleotides | TransIT®-Oligo Transfection Reagent |
| Plasmid DNA and oligonucleotides | TransIT-X2® Dynamic Delivery System and TransIT®-Oligo Transfection Reagent |
| Other combinations | Contact techsupport@mirusbio.com |
If a single transfection reagent is not suitable for a specific co-delivery application, then separate transfection complexes can be prepared using the optimal transfection reagent for each nucleic acid and added to the cells at the same time. As long as the individual transfection protocols are compatible, this method would ensure uptake of all nucleic acids by the cell simultaneously. An example is the co-delivery of plasmid DNA and mRNA that is explained in detail here.
![]() Don’t See Your Cell Type? Consult Reagent Agent® Transfection Database
Don’t See Your Cell Type? Consult Reagent Agent® Transfection Database
Citation Database: Check if our reagents have been used by other researchers to transfect your cell type
Technical Support: Speak directly with a transfection expert