Mirus Bio receives ISO 13485:2016 certification, underscoring the quality of processes used to support GMP product portfolio. Read more
Mirus Bio receives ISO 13485:2016 certification, underscoring the quality of processes used to support GMP product portfolio. Read more
Completion of this comprehensive certification process underscores the quality of the processes used to support the company’s GMP product portfolio
View the certificateVirusGEN® is a proven transfection reagent combining industry-leading yield performance with real world economic
advantages by delivering a reduced cost per dose — all supported by comprehensive expertise for cell and gene therapy.
VirusGEN® specifications support virus production fit-for-purpose requirements.
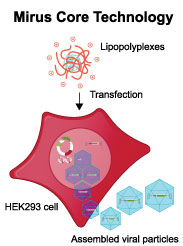 What makes VirusGEN® unique?
What makes VirusGEN® unique?
What benefit does this bring?
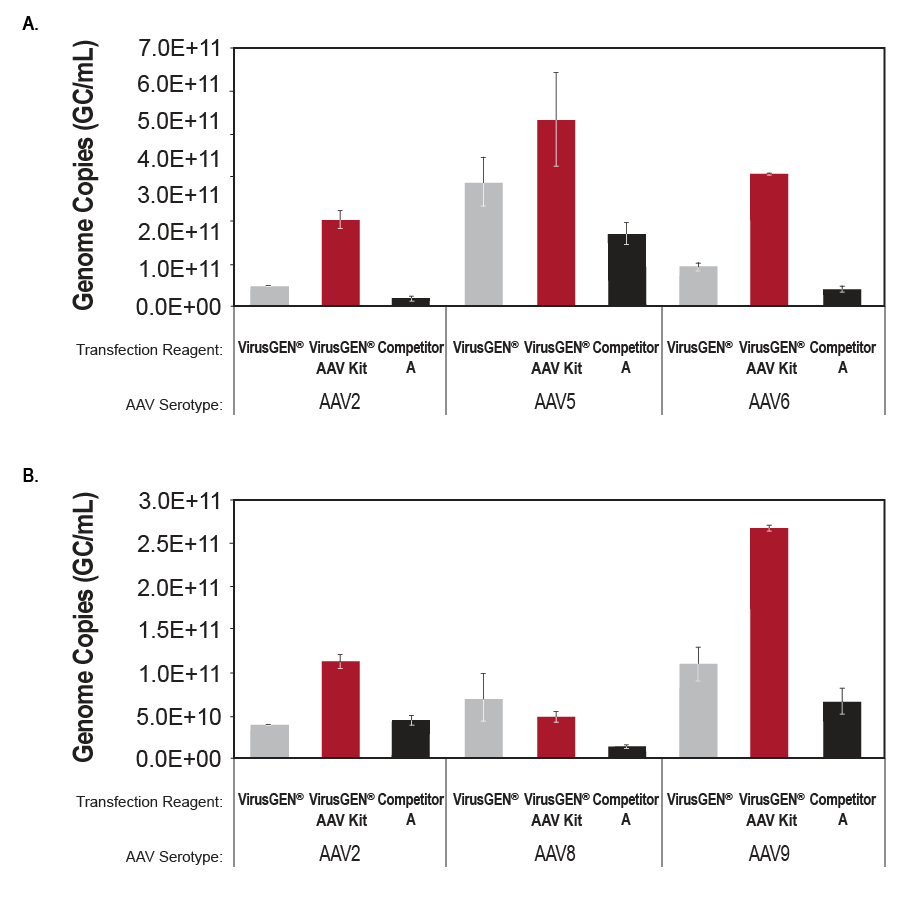
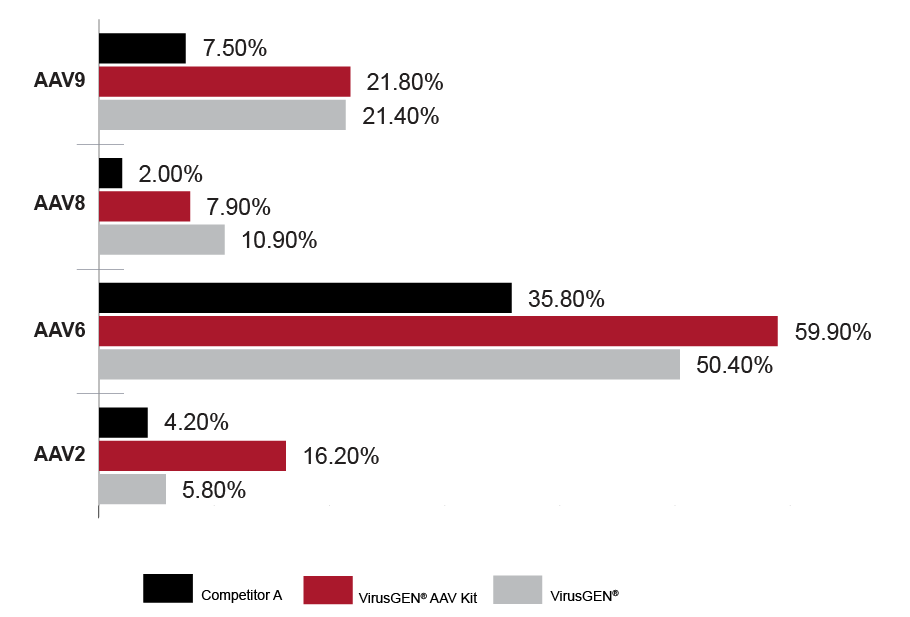
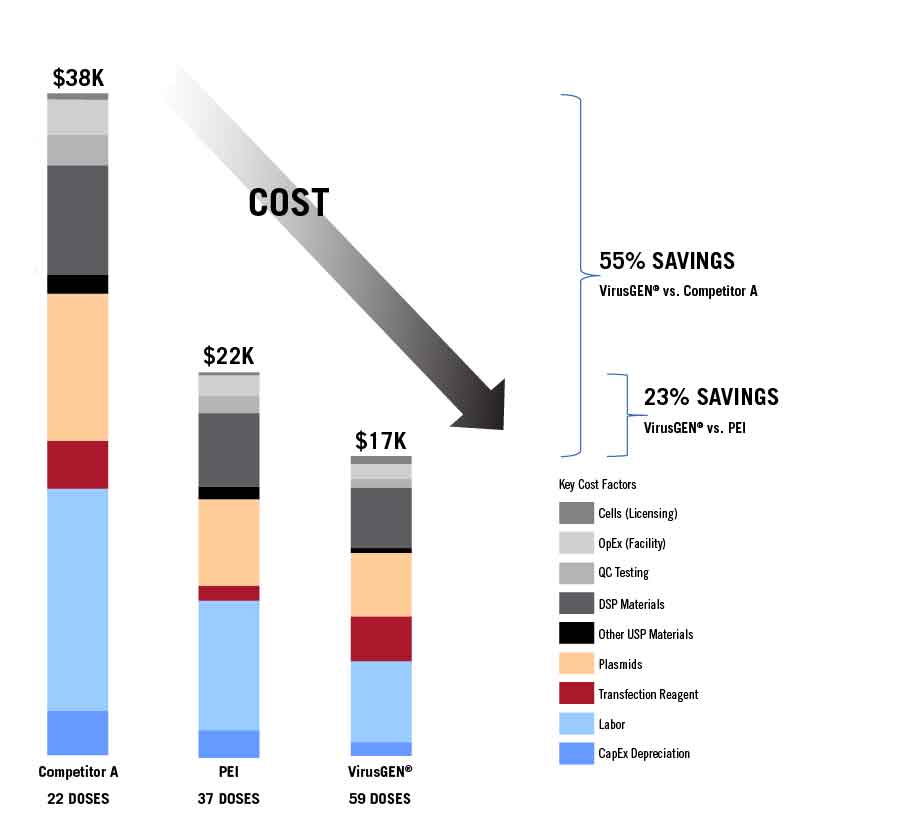
Specifications
Storage Conditions:
VirusGEN® GMP AAV Transfection Kit: Multiple storage conditions – see individual bottles for specific recommendations
TransIT-VirusGEN® GMP Transfection Reagent: Store at -10 to -30°C
Product Guarantee:
All Configurations: Refer to Certificate of Analysis for Retest Date
Usage Statement:
All Configurations: For Research Use and Further Manufacturing; Not for Administration into Humans.
Animal Origin Statement:
All Configurations: This product is animal origin free.
Directly From Our Customers
![]() Listen to the first-hand account of how Mirus helped researchers from St. Jude overcome the challenges with viral vector optimization to produce ground-breaking gene therapy for hemophilia. Go to the podcast >>
Listen to the first-hand account of how Mirus helped researchers from St. Jude overcome the challenges with viral vector optimization to produce ground-breaking gene therapy for hemophilia. Go to the podcast >>