Mirus Bio receives ISO 13485:2016 certification, underscoring the quality of processes used to support GMP product portfolio. Read more
Mirus Bio receives ISO 13485:2016 certification, underscoring the quality of processes used to support GMP product portfolio. Read more
Recombinant protein and mAb production
Get the titer you need from your cell line of choice
We’ve designed an advanced transfection solution for industry-leading protein titers from suspension CHO cells. Our CHOgro® transfection system has everything you need to reach high antibody titers, faster. It includes scalable expression medium, complex formation solution, our powerful TransIT-PRO® Transfection Reagent, and other components for convenient and affordable protein production that is adaptable to a variety of CHO cell lineages.
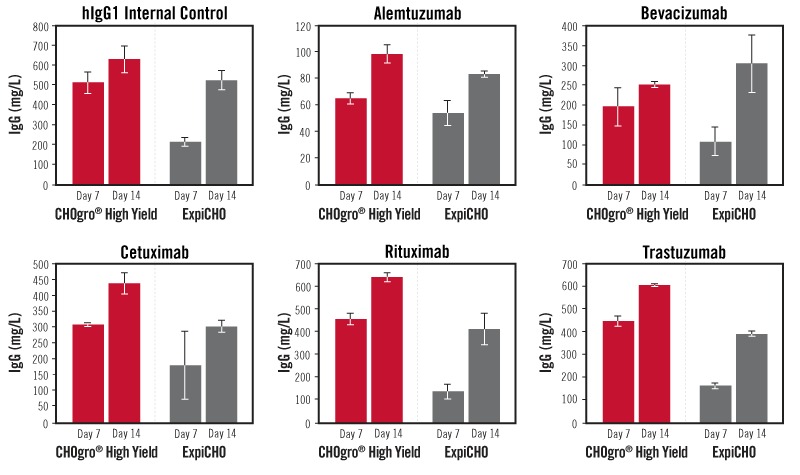
The CHOgro® High Yield Expression System Outperforms the ExpiCHO Expression System Using Multiple Antibody Constructs. Six different IgG1 antibody constructs, including five therapeutically relevant constructs synthesized in the same vector backbone were produced by transient transfection using either the TransIT-PRO® Transfection Reagent (Mirus Bio) at a 1:1 reagent-to-DNA ratio (vol:wt) and 1 µg plasmid DNA per milliliter of culture in FreeStyle™ CHO-S cells (ThermoFisher Scientific) cultured in CHOgro® Expression Medium (Mirus Bio) at a cell density of 4 x 106 cells/ml, or the Expifectamine CHO Transfection Kit (ThermoFisher Scientific) at a 3.2:1 reagent-to-DNA (vol:wt) and 1 µg plasmid DNA per milliliter of culture in ExpiCHO-S cells (ThermoFisher Scientific) cultured in ExpiCHO Expression Medium (ThermoFisher Scientific) at a cell density of 6 x 106 cells/ml. All cells were split into 125 ml Optimum Growth flask (25 ml/flask, Thomson Instrument Company) at the time of transfection. The CHOgro® Titer Enhancer was added immediately post-addition of transfection complexes and the culture was incubated at 32°C shaking until harvest for the CHOgro® High Yield Expression System. The Max titer protocol was followed for the ExpiCHO Expression System (ThermoFisher Scientific): Expifectamine CHO Enhancer and Feed (ThermoFisher Scientific) were added at 24 hours post-transfection and cultures were shifted to 32°C. At Day 5 a second volume of the ExpiCHO Feed (ThermoFisher Scientific) was added to the flask. Day 7 and 14 supernatants were analyzed using a standard sandwich human IgG ELISA. Error bars represent the standard deviation of triplicate technical replicates.
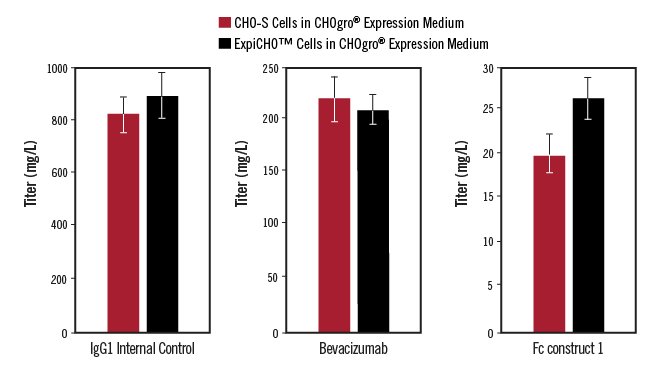
FreeStyle™ CHO-S and ExpiCHO Cells Grown in CHOgro® Expression Medium Yield Similar Titers. FreeStyle™ CHO-S cells (ThermoFisher Scientific) or ExpiCHO-S cells (ThermoFisher Scientific) were cultured in CHOgro® Expression Medium (Mirus Bio). Both cell lines were transiently transfected with plasmid DNA encoding a human IgG1 internal control antibody, Bevacizumab or Fc-fusion construct using the TransIT-PRO® Transfection Reagent (Mirus Bio) at a 1:1 reagent-to-DNA ratio (vol:wt) and 1 µg plasmid DNA per milliliter of culture in a non-treated 6-well plate. All cultures were shifted to 32°C, shaking, immediately post-addition of the transfection complexes and the CHOgro® Titer Enhancer to the culture. Day 14 supernatants were analyzed using a standard sandwich human IgG ELISA. Error bars represent the standard deviation of triplicate technical replicates.
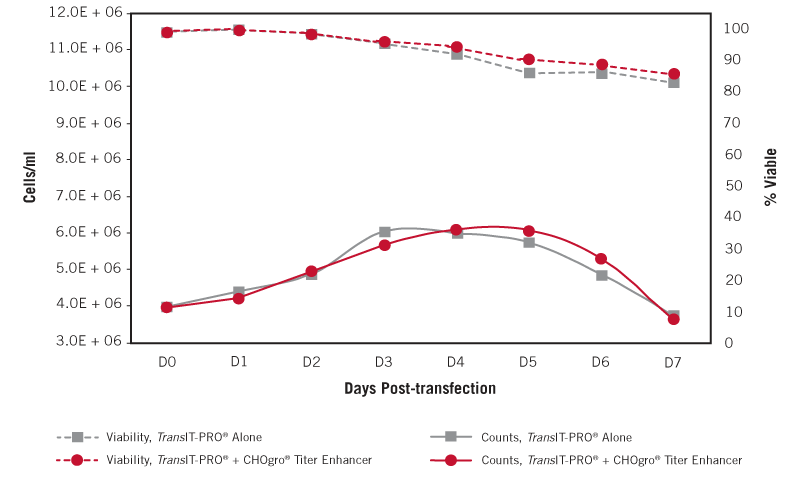
CHOgro® Titer Enhancer Does Not Adversely Affect Cell Growth and Viability Post-transfection. Triplicate flasks of FreeStyle™ CHO-S cells adapted to CHOgro® Expression Medium were transiently transfected with the TransIT-PRO® Transfection Reagent (Mirus Bio) at a 1:1 reagent-to-DNA ratio (vol:wt) and 1 µg plasmid DNA per milliliter of culture at 4 x 106 cells/ml in 125 ml Optimum Growth flasks (25 ml/flask, Thomson Instrument Company). As indicated, CHOgro® Titer Enhancer was added and all of the cultures were shifted to 32°C immediately post-addition of the transfection complexes to the culture. Cell counts (solid line) and viability (propidium iodide staining, dotted line) were measured daily post-transfection using a Guava® easyCyte™ 5HT flow cytometer (EMD Millipore).
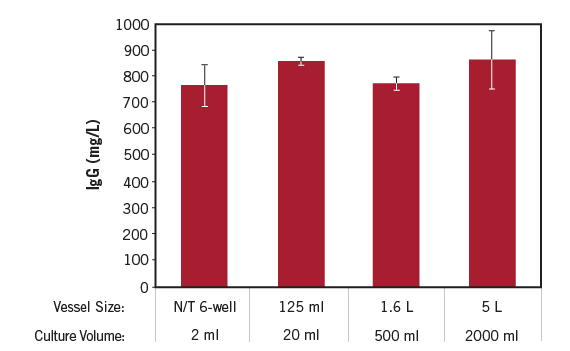
CHOgro® High Yield Expression System Enables 1000-fold Scalability. Human IgG1 was produced by transient transfection with the TransIT-PRO® Transfection Reagent (Mirus Bio) and 1 µg plasmid DNA per milliliter of culture at a 1:1 reagent:DNA ratio. Freestyle CHO cells (Thermo Fisher Scientific) were transfected at a density of 4 x 106 cells/ml in CHOgro® Expression Medium (Mirus Bio) at the following volumes/culture vessels: 2 ml/non-tissue culture treated 6-well dish, 20 ml/125 ml Thomson flask, 500 ml/1.6 L Thomson flask, 2000 ml/5 L Thomson flask. All cultures were shifted to 32°C, shaking, immediately post-addition of the transfection complexes and the CHOgro® Titer Enhancer to the culture. Cultures were maintained at the appropriate shake speed for the remainder of the experiment. Day 14 supernatants were analyzed using a standard sandwich human IgG ELISA. Error bars represent the standard deviation of triplicate technical replicates.
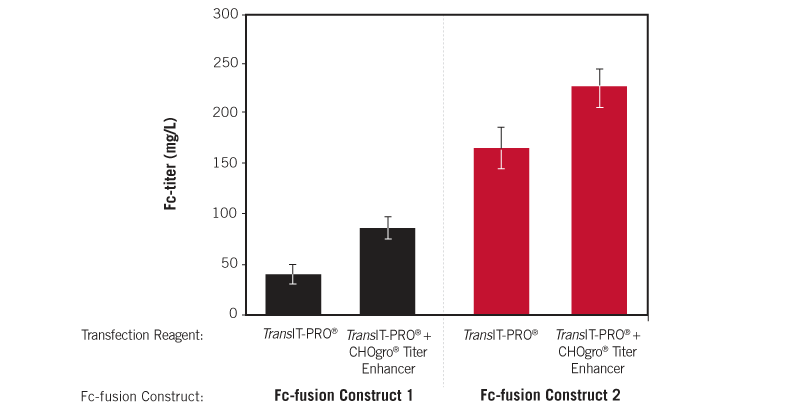
Titer Enhancement is Observed Using Multiple Fc-fusion Constructs. Two different Fc-fusion constructs were transiently transfected using the TransIT-PRO® Transfection Reagent (Mirus Bio) at a 1:1 reagent-to-DNA ratio (vol:wt) and 1 µg plasmid DNA per milliliter of culture. FreeStyle™ CHO-S (ThermoFisher Scientific) cells were cultured in CHOgro® Expression Medium (Mirus Bio) and plated at 4 x 106 cells/ml at the time of transfection in non-treated 6-well plates (2 ml/well). As indicated, CHOgro® Titer Enhancer was added and the culture was shifted to 32°C immediately post-addition of the transfection complexes to the culture. Day 10 supernatants were analyzed using a standard sandwich human IgG ELISA. Error bars represent the standard deviation of triplicate technical replicates.
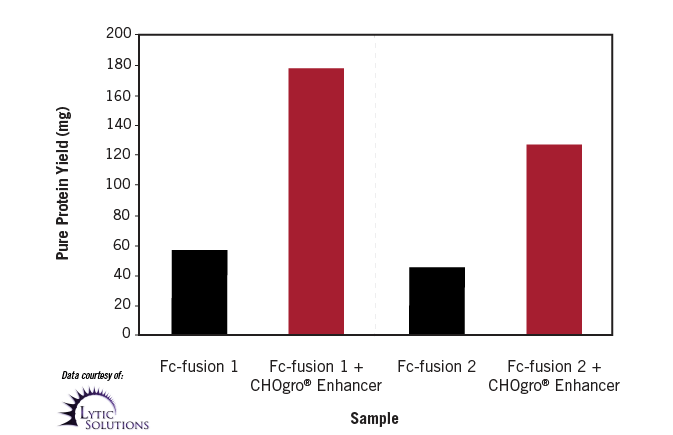
Higher Titers Achieved by Third Party with CHOgro® High Yield Expression System Plus CHOgro® Titer Enhancer. Fc-fusion proteins were produced by transient transfection of CHO-S cells in complete CHOgro® Media using TransIT-PRO® Transfection Reagent (1:1 reagent:DNA ratio, volume: weight). Cells were either 2 x 106 cells/ml (no CHOgro® Titer Enhancer) or 4 x 106 (with CHOgro® Titer Enhancer) for transfection. Cultures were shifted to 32°C either 24 hours (no CHOgro® Titer Enhancer) or immediately (with CHOgro® Titer Enhancer) following transfection. Yields represent final affinity-purified protein from 0.5 L culture, harvested at day 11 post-transfection.
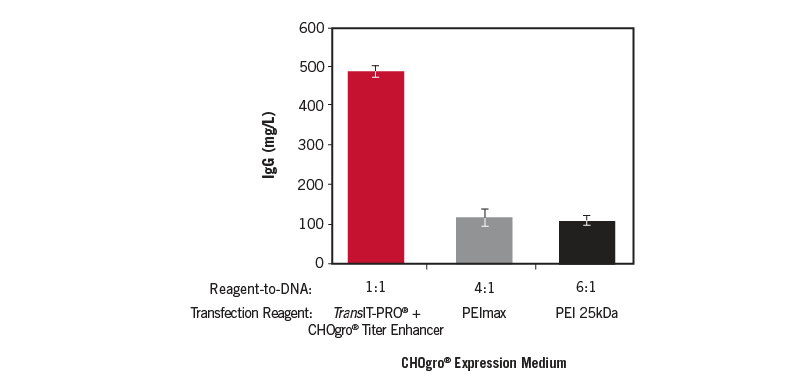
TransIT-PRO® + CHOgro® Titer Enhancer Outperforms PEImax and linear 25 kDa PEI Transfection Reagents. An IgG1 antibody construct was produced by transient transfection using either the TransIT-PRO® Transfection Reagent (Mirus Bio) at a 1:1 reagent-to-DNA ratio (vol:wt), PEImax (4:1, Polysciences), or linear 25 kDa PEI (6:1, Polysciences) and 1 µg plasmid DNA per milliliter of culture in FreeStyle™ CHO-S cells (ThermoFisher Scientific) cultured in CHOgro® Expression Medium (Mirus Bio) at a cell density of 4 x 106 cells/ml. CHOgro® Titer Enhancer (Mirus Bio) was added immediately post-addition of transfection complexes and cultures were incubated at 32°C with shaking until harvest for all reagents. Day 14 supernatants were analyzed using a standard sandwich human IgG ELISA. Error bars represent the standard deviation of triplicate technical replicates.
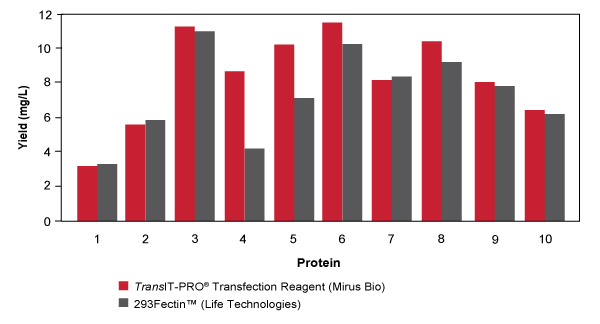
Achieve High Protein Yields Using TransIT-PRO® Transfection Kit in Suspension 293 Cells. Ten different secreted (non‐antibody) proteins were transiently expressed in FreeStyle™ 293‐F cells (Life Technologies) using TransIT‐PRO (1.5:1) or 293fectin™ (Life Technologies, 2:1) transfection reagents according to manufacturer’s protocol. Cells were grown in FreeStyle™ 293 Expression Medium at transfected at a density of 1 x 106 cells/ml. The scale of the transfection for each protein varied between 1‐6 L of culture. More experimental details here. (PDF)
Data courtesy of a TransIT‐PRO pharmaceutical customer.
| Antibody Expression | Scale(L) | Yield(mg/L) |
|---|---|---|
Eight different antibodies were transiently expressed in FreeStyle™ 293‐F cells (Life Technologies) using the TransIT‐PRO Transfection Reagent (1:1) according to manufacturer’s instructions. Cells were grown in FreeStyle™ 293 Expression Medium and transfected at a density of 1 x 106 cells/ml. The scale of the transfection for each protein varied between 1 ‐ 11 L of culture. Data courtesy of a TransIT‐PRO customer from a Contract Research Organization (CRO). | 1 | 18.1 |
| 5 | 15.4 | |
| 6 | 25.0 | |
| 6 | 11.2 | |
| 6 | 20.0 | |
| 5 | 18.8 | |
| 10 | 13.3 | |
| 11 | 33.3 |
Expi293™ and Expi293F™ are trademarks of Life Technologies Corporation.
Download Protocol: TransIT-PRO® Transfection Reagent for EXPI293F™ Cells (PDF)










